Chapter 13 Post Submission Tips
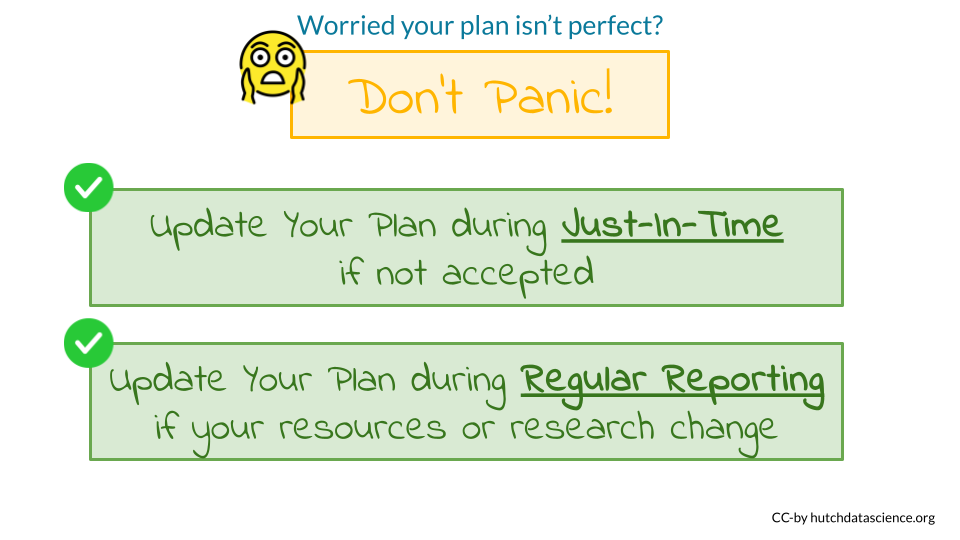
In this section we will discuss tips about what might be needed after you submit your DMS plan:
- You can update your DMS plan if is not accepted during the Just-in-Time process.
- You can also update your DMS plan during regular reporting intervals, but these changes will need to be approved by program staff. Researchers are encouraged to discuss such changes in advance.
- Examples of appropriate changes include:
- change in type of data generated
- a more appropriate data repository becomes available
- sharing timeline shifts
- Examples of appropriate changes include:
- Investigators are expected to report progress made on data sharing in their annual Research Progress Performance Report (RPPR).
- NIH staff will monitor compliance with the DMS plan during this process.